Protein Gel
Dominik Haudenschild 11/93
at CBRCPour Gel
Prepare plates and gel unit. Clean. Ensure that plates don't leak.
Prepare lower gel
• Determine proper gel concentration and prepare lower buffer in 15mL centrifuge tube.
• Mix all reagents except TEMED and Ammonium persulfate.
• Proceed to the next few steps carefully and quickly:
• Add proper amount of TEMED to middle of solution.
• Invert 4 times.
• Add Ammonium persulfate.
• Invert 4 times.
• Pipette 3.75 mL between plates on gel holder. Avoid air bubbles. Air bubble will ruin gel.
• Carefully pipette water over top of gel to help polymerization and to avoid meniscus formation of gel.
|
SDS Gel preparation table. |
||||||||||||||||||||
|
Gel % |
||||||||||||||||||||
|
Upper |
Lower |
|||||||||||||||||||
|
Solution |
4 |
5 |
6 |
7 |
8 |
10 |
12 |
15 |
20 |
|||||||||||
|
30% Acrylimade stored at 4 degrees (mL) |
1.0 |
2.5 |
3.0 |
3.5 |
4.0 |
5.0 |
6.0 |
7.5 |
10.0 |
|||||||||||
|
Lower Buffer (mL) |
3.75 |
3.75 |
3.75 |
3.75 |
3.75 |
3.75 |
3.75 |
3.75 |
||||||||||||
|
Upper Buffer (mL) |
1.88 |
|||||||||||||||||||
|
10 % SDS (無) |
75 |
150 |
150 |
150 |
150 |
150 |
150 |
150 |
150 |
|||||||||||
|
Water (mL) |
4.58 |
8.50 |
8.00 |
7.50 |
7.00 |
6.00 |
5.00 |
3.53 |
1.02 |
|||||||||||
|
Add Last |
||||||||||||||||||||
|
TEMED (無) |
7.5.無 |
7.5 無 |
7.5無 |
7.5 無 |
7.5 無 |
7.5 無 |
7.5 無 |
7.5 無 |
7.5 無 |
|||||||||||
|
10% AmPS (無) |
75.0 無 |
75.0 無 |
75.0 無 |
75.0 無 |
75.0 無 |
75.0 無 |
75.0 無 |
75.0 無 |
75.0 無 |
|||||||||||
|
Total volume |
7.5mL |
15mL |
15mL |
15mL |
15mL |
15mL |
15mL |
15mL |
15mL |
|||||||||||
• Wait 15 minutes till polymerized.
• Remove water from top of gel.
•Wash top of lower gel twice with water to remove any unpolymerized solution.
• Dry top of gel and plates with edge of paper towel.
Top Stacking Gel
• After lower gel has finished, prepare top stacking gel.
• Mix solutions for upper gel as was done for lower gel. Note differences in volumes. Mix as before, by adding TEMED and ammonium persulfate last.
• Pour in top gel, with proper comb, using bulb pipette at a moderate yet consistent pace; avoid air bubbles.
• Polymerize 30 minutes.
Gel Box
• Snap gel aparatus together and prepare gel box. Ensure that containers don't leak.
• Pour gel buffer into middle of aparatus and outside of appartus. Do not let inside and outside solutions mix. Buffer is 1x Tris/Glycine/SDS Buffer (10x stock from BIO RAD Cat: 161-0732) in water.
Prepare samples
• Determine proper amount of samples to use. Use 痢rams per well -- amount will vary with sample type. Thin 15 well comb holds 15無 comfortably. Add sample buffer to be 1x (Stock sample buffer is 4x). Use appropriate volume of water to achieve final volume. A total sample size of 5無 to 15無 works well.
Ex: Use 1 to 5痢 sample (possibly 1 無 at a 5mg/mL concentration) + 1.25無 sample buffer (1/4 of final volume of 5無) and adjust to 5 無 with water.
• Prepare appropriate well markers. Use high, low, or broad markers. Dilute Biorad markers 1:40 in 1x sample buffer. A 250 無 stock solution of ready to use marker can be made
6.25 無 Stock marker 40x.
62.5 無 sample buffer 4x.
231.25 無 water.
Store at -20
oC.• Heat samples at 95°C for 5 minutes.
Run Gel
• Load samples into gel using gel loader tips. Avoid cross contamination. Note where each samples is placed.
• Run gel at 25mA per gel for an hour or so. Let dye front run to almost bottom of plate. Nothing runs faster than that front.
Transfer
• Prepare transfer buffer. Towbins recommended.
Buffer is 1x TG buffer with 15% methanol Buffer.
1500 mL is sufficient for two gels.
• Remove gel onto plate with such that the gel is on top of the plate and lane 1 is at the upper left hand corner.
• Submerge large plate with gel on it into transfer buffer. Total time around 10 minutes.
• Cut membranes (Immobilon-P Transfer Membrances from Millipore) size of small plate.
• Submerge membrane in methanol.
• Place in water until membrane submerges.
• Place membranes in Towbins buffer for 10 minutes.
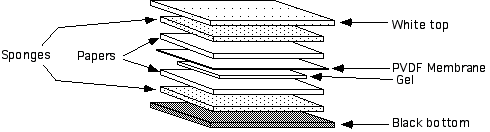
• Build transfer apparatus. Black side down. Sponge, Paper, Gel, Membrane, Paper, Sponge and close white side (sometimes red) down. 
• Set in transfer gel box filled with Towbins buffer.
• Run transfer at 4
oC with ice pack, and stirring.• Set at 22 volts over night
• Turn to 76 for
• Set at 90 volts for 1 hour. Change ice pack after
1/2 hour.Coomassie Blue Staining
• Remove gel and membranes from holders.
Stain Membrane
• Place membrain in coomassie blue stain for 5 min.
• Put in destain ( 40 to 60% Methanol 5% Glacial acetic acid). Stain membrane until most of background is gone. Do not destain too long; it will whiten upon placement into water and drying.
• Place in water quickly to wash off destain.
• Dry quickly on paper towel.
• Photocopy for future reference. Clean copier so that fingerprint oils on copier glass don't affect membrane staining.
Stain Gel
• Place gel in coomassie blue stain .
• Stain 1
1/2-2 min in microwave.• Stain additionaly 30 min-2hr.
• Destain in destain (7.5% CH
3OH, 7.5% CH3COOH) with paper towels until gel is clear, and only bands are blue.• Seal in cellophane or harden in acetone.
Antibody Staining
• Destain completely in methanol for 5 min to completely remove stain.
• Wash in water to remove methanol.
• Wash in PBS.
• Meanwhile prepare 1%BSA. in PBS
• Block in 1% BSA in PBS(or3% DMP or Zeller's solution) for
1/2 hour to block or O/N at 4oC.• Meanwhile prepare antibody solutions. Dilute antibody in blocking solution or PBS Tween. For dilution: 1mL 3% DMP in PBS + 3 mL of PBS Tween works well.
• Place membrane in bag with almost no extra space, add antibody solution, seal without air bubbles, and place on rocker for 30 min. 3-4 mLs of antibody solution should be sufficient for a small bag.
• Wash in PBS -Tween 5 times 5 min. each. Larger volumes wash better.
• Wash in secondary antibody, in a bag for 30 min. 3-4 mL of secondary should be prepared for each membrane. Use 3-4 mL of PBS Tween and add appropriate secondary antibody (HRP a-mouse or a-rabbit) diluted 1:3000.
• Wash in PBS -Tween 5 times 5 min. each. Pipette tip cover.
Illuminate Film
• Mix 3mL A + 3 mL B of Western Blot Chemiluminesence reagents (ECL).
• Wash membrane in reagent mixture for 1 min.
• Bring to dark room.
• Put gel on well cleaned scratch free plexi glass, or transparency.
• Put overhead transparency form on top.
• Expose film (Kodak X-OMat). Time varies from 1sec -> 1min. 10 sec recomended as trial.
• Develop.
• Repeat exposure until suitable developed film results.
Solutions
SDS-PAGE Stock Solutions
Monomer Stock 30% Acrylamide
29.2g Acrylamide (37.5:1 ratio)
0.80g Bis
-Bring Volume to 100ml with Milli-Q water
-Store in dark at 4'C
Alternative: Buy BMB #100681 premixed, $50
Separating Gel Buffer, 4X
36.3g Tris Base =1.5M
-pH to 8.8 with Concentrated HCl
-Bring volume to 200ml with Milli-Q water
-Store at 4'C
Stacking Gel Buffer, 4X
6.0g Tris Base =0.5M
-pH to 7.0 with concentrated HCl
-pH to 6.8 with 1M HCl
-Bring volume to 100ml with Milli-Q water
-Store at 4°C
Tank Buffer, 10X
15.0g Tris Base =
72.0g Glycine =
50ml 10%SDS
-Bring volume to 500ml with Milli-Q water
-Check pH of 1X solution should be 8.4
SDS is a very fine powder that is extremely irritating to lungs, eyes, and skin. Always wear dust mask, glasses and gloves. Turn off nearby fans or fume hoods (if possible). Wipe clean all surfaces that may have been contaminated.
When working with Acids wear gloves and glasses.
4x Sample buffer
To 50 mL tube add:
17.9 mL 8.9M deionized urea = 8M
1.21 g Tris base = 0.5M
• pH to 6.8 with concentrated HCL then add:
3.2 g SDS
200 無 96 mg/mL bromophenol blue = 0.096%
• Bring volume to 20.0 mL with Milli-Q water.
• Mix well
• Allow suds to settle (may take overnight)
• Store 500無 aliquots at -20
10% SDS in Water
To 70ml Milli-Q water add slowly
10.0g SDS (Sodium Dodecyl Sulfate)
-Gently stir without forming bubbles
-Do not heat solution
-Bring volume to 100 ml with Milli-Q water
SDS is a very fine powder that is extremely irritating to lungs, eyes, and skin. Always wear dust mask, glasses and gloves. Turn off nearby fans or fume hoods (if possible). Wipe clean all surfaces that may have been contaminated.
Notes
SDS is a very fine poweder that is extremely irritating to lungs, eyes and skin. Always wear dust mask, glasses and gloves. Turn off nearby fans or fume hoods (if possible). Wipe clean all surfaces that may have been contaminated.
When working with acids wear gloves and glasses.
Acrylamide is a toxic material; wear gloves, glasses, and dust mask. Avoid generating dust. Wipe all surfaces that may be contaminated.